- What is an electrolyzer test stand, and how does it differ from a full-stack test setup or a production-grade system?
- Why is it important to study ageing in electrolyzer cells, and what impact does it have performance-, efficiency-, and durability-wise?
- How does ageing occur in electrolyser cells, and what are its common root causes (e.g. catalyst degradation, membrane thinning, delamination)?
- What does “in-situ diagnostics” mean in the context of electrolyser testing, and why is it critical for understanding ageing processes?
- How is electrochemical impedance spectroscopy (EIS) integrated into the test setup, and what additional insights does it provide?
What is an electrolyzer test stand, and how does it differ from a full-stack test setup or a production-grade system?
Electrolyzer test stands are specialized experimental setups used to investigate the production of hydrogen (H₂) and oxygen (O₂) using electric current. These electrolyzer test stands can range in scale from individual cells to full-scale stacks. The primary goal is to evaluate and optimize the performance of the electrolyzer cell/stack under conditions that closely mirror real-world operation. To achieve this, the test stand allows for controlled variation of key parameters such as pressure, temperature, concentrations, and reactants, while keeping other conditions constant. This makes it possible to systematically study cause-effect relationships and generate targeted know-how. In particular, it enables researchers to test different materials, component designs, and operating strategies.
Depending on the focus, test stands may serve either research and development (R&D) purposes, producing only small amounts of hydrogen, or be tailored toward industrial validation, where larger volumes of hydrogen and oxygen are generated under near-production conditions.
In contrast to full-stack or production-grade systems that prioritize efficiency and output, test stands emphasize flexibility, modularity, and diagnostic access. This makes them critical for early-stage innovation and component-level validation.
Why is it important to study ageing in electrolyzer cells, and what impact does it have performance-, efficiency-, and durability-wise?
Cell ageing has a direct impact on electrolyzer performance. It refers to the gradual degradation of materials within the cell over time due to chemical and physical stressors. This degradation manifests in increased ohmic resistance, higher concentration losses, and greater activation losses. As a result, the cell produces less hydrogen (H₂) and consumes more energy.
Through ageing analysis, researchers can examine how specific materials behave under operating conditions and identify weak points. These insights enable targeted improvements in future cell designs, ultimately extending operational lifetime. The most common ageing effects include:
- Catalyst degradation
- Membrane thinning
- Increased ohmic resistance
- Gas crossover
- Electrode corrosion
- Mechanical failure
Understanding the root causes of these phenomena is essential for enhancing system durability and efficiency; both of which are critical for making green hydrogen economically viable and competitive with conventional fossil fuels.
Early identification of ageing mechanisms not only improves long-term system performance but also enables predictive maintenance strategies, reducing unplanned downtime in commercial applications.
How does ageing occur in electrolyser cells, and what are its common root causes (e.g. catalyst degradation, membrane thinning, delamination)?
Membrane thinning represents a major failure mode in electrolysis systems. Membrane thinning and structural weakening can result from heat buildup caused by high current densities or inadequate cooling, as well as from mechanical stresses such as pressure changes and repeated swelling-contraction cycles. Over time, these stresses may compromise membrane integrity. In the worst-case scenario, a hole in the membrane may develop, which can result in a hydrogen-oxygen (oxyhydrogen) reaction or mixing of the electrolytes. Such events can lead to irreversible damage to the system.
Catalyst degradation in electrolyzers leads to a loss of efficiency over time. The anode is especially affected, as the oxygen evolution reaction (OER) takes place there, often causing catalyst corrosion, particularly under high voltages and in acidic environments. Mechanical stress, such as gas bubble formation, can also detach particles and further reduce performance.
Another ageing factor includes delamination of electrodes from the membrane, often due to differential thermal expansion or long-term exposure to aggressive chemical conditions. This can lead to increased contact resistance and local hot spots.
What does “in-situ diagnostics” mean in the context of electrolyser testing, and why is it critical for understanding ageing processes?
In-situ measurement refers to observing and analyzing parameters inside the cell during real-time operation.
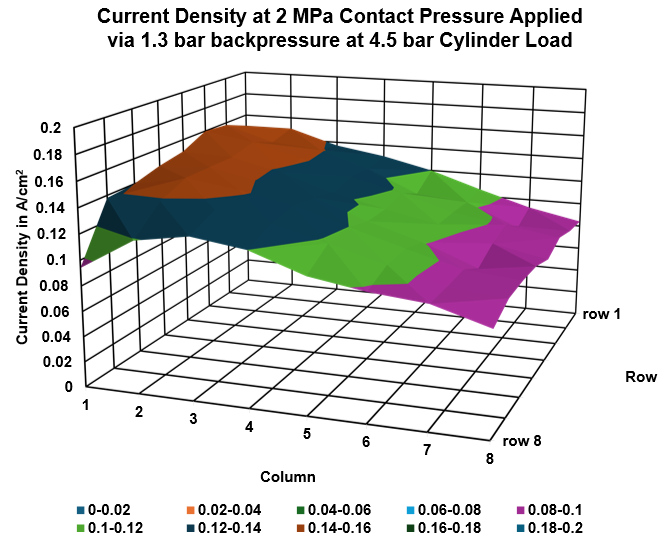
Using specialized diagnostic tools, it is possible to gain insights directly within the cell, enabling the monitoring of key parameters such as:
- Current density distribution
- Temperature gradients
- Homogeneity
This allows researchers and engineers to detect and track cell aging effects as they occur under realistic operating conditions, providing valuable data for understanding degradation mechanisms and improving long-term performance and durability. Unlike post-mortem analysis, in-situ diagnostics capture transient behaviours and dynamic responses, making them especially useful for intermittent renewable energy applications where electrolyzers face frequent load changes.
How is electrochemical impedance spectroscopy (EIS) integrated into the test setup, and what additional insights does it provide?
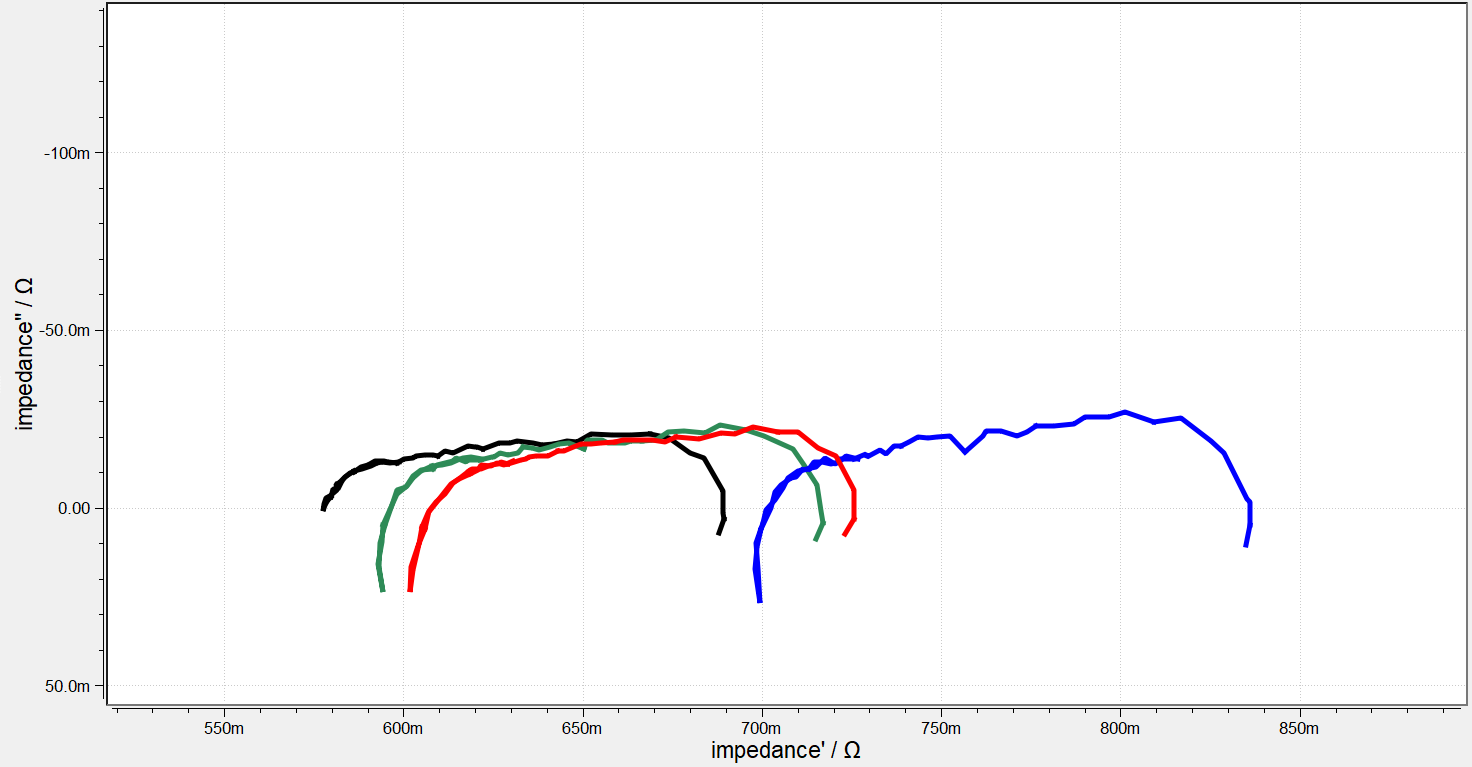
Electrochemical Impedance Spectroscopy (EIS) is a powerful diagnostic tool that enables the separation of a cell’s total impedance into its fundamental components. These include:
- Diffusion-related phenomena (mass transport)
- Charge transfer kinetics at the electrodes
- Ohmic resistance from the membrane and contact interfaces
By decoupling these effects, EIS provides detailed insights into the limiting processes and degradation mechanisms within the cell.
This analytical capability allows each mechanism to be studied independently, offering material scientists a deeper understanding of how components behave under real operating conditions. A particularly valuable advancement is segmented EIS, which enables localized analysis. Using this method, spatial phenomena such as membrane dehydration (starvation) or flooding in flow field regions can be detected and precisely mapped.
These insights are highly beneficial for electrolyzer manufacturers and system operators. With a clearer picture of internal cell behavior, systems can be optimized for greater efficiency; particularly under dynamic operating conditions, as required in renewable energy applications. In such contexts, power input is inherently fluctuating due to the variable nature of sources like wind and solar. As a result, electrolyzers must frequently ramp up, shut down, or operate under partial loads far from steady-state conditions making robust diagnostics like EIS essential for ensuring long-term performance and durability.
EIS can also aid in validating simulation models by providing empirical data on internal resistances and response behavior, bridging the gap between theoretical design and practical implementation.
Content contributed by DILICO engineering GmbH
DILICO engineering develops and produces measurement technology for monitoring and analysing electrolysis cells, fuel cells, redox flow batteries and batteries. This includes cell voltage monitoring and contacting solutions for cell voltage pick-up, in-situ current and temperature distribution measurements as well as EIS measurements for ageing analysis and evaluation of operational management. Electrolysis test stands for the reproducible measurement and analysis of electrolysis single cells are another of DILICO's areas of expertise.













