Browse fuel cell systems from leading suppliers on our marketplace!
How does a fuel cell work?
A fuel cell is a device that converts the chemical energy of hydrogen into electricity through an electrochemical reaction. Fuel cells can be used as a source of electricity for a wide range of applications, including vehicles, portable power generators, and stationary power systems.
The basic fuel cell parts are an anode, a cathode, and an electrolyte membrane. Hydrogen gas is supplied to the anode, where it is split into protons and electrons. The protons pass through the electrolyte membrane, while the electrons are forced to travel through an external circuit, creating an electric current. At the cathode, the protons and electrons are reunited with oxygen from the air to produce water and heat as byproducts.
How are fuel cells made?
The make-up and materials used to manufacture specific types of fuel cells depend very much on the type of fuel and oxidant used to generate electrical energy. As a matter of principle, every fuel cell consists of anode, cathode, and electrolyte. These are designed in multiples, in sets of thin layers, generally using a membrane. The idea is to maximize the surface area allowing the chemical reaction to occur. The membranes are made of material adapted to specific fuel cell types, based on the fuel used – therefore based on the actual chemical reaction taking place.
Platinum in fuel cells
Platinum plays an important role in fuel cell engineering. This inert metal is often used as a catalyst in fuel cells, particularly in PEM (proton exchange membrane) fuel cells, to facilitate the electrochemical reactions that occur at the anode and cathode. The platinum fuel cell membrane coating represents an effective catalyst due to its high surface area and ability to facilitate the transfer of electrons between the hydrogen molecules and the oxygen molecules.
However, platinum is also a relatively expensive and scarce resource, which is why current research on fuel cells is focused on reducing the amount of platinum used in fuel cells, including:
- Using alternative catalysts: Researchers are exploring the use of alternative catalysts, such as cobalt, iron, and nickel, which may be able to perform the same functions as platinum but at a lower cost.
- Developing new catalyst materials: Researchers are also working on developing new catalyst materials that are more efficient and require less platinum.
- Developing new catalyst designs: Researchers are investigating new designs for catalysts that may be able to operate more efficiently and require less platinum.
Overall, the use of platinum in fuel cells is likely to continue to be a topic of research and development, as engineers strive to improve the efficiency and cost-effectiveness of fuel cell technologies.
Fuel cell technologies
The chemistry of fuel cells is at the heart of fuel cell design. Every design is based fundamentally on a specific electrochemical fuel cell reaction (with the exception of a so-called microbial fuel cell, or enzymatic biofuel cell).
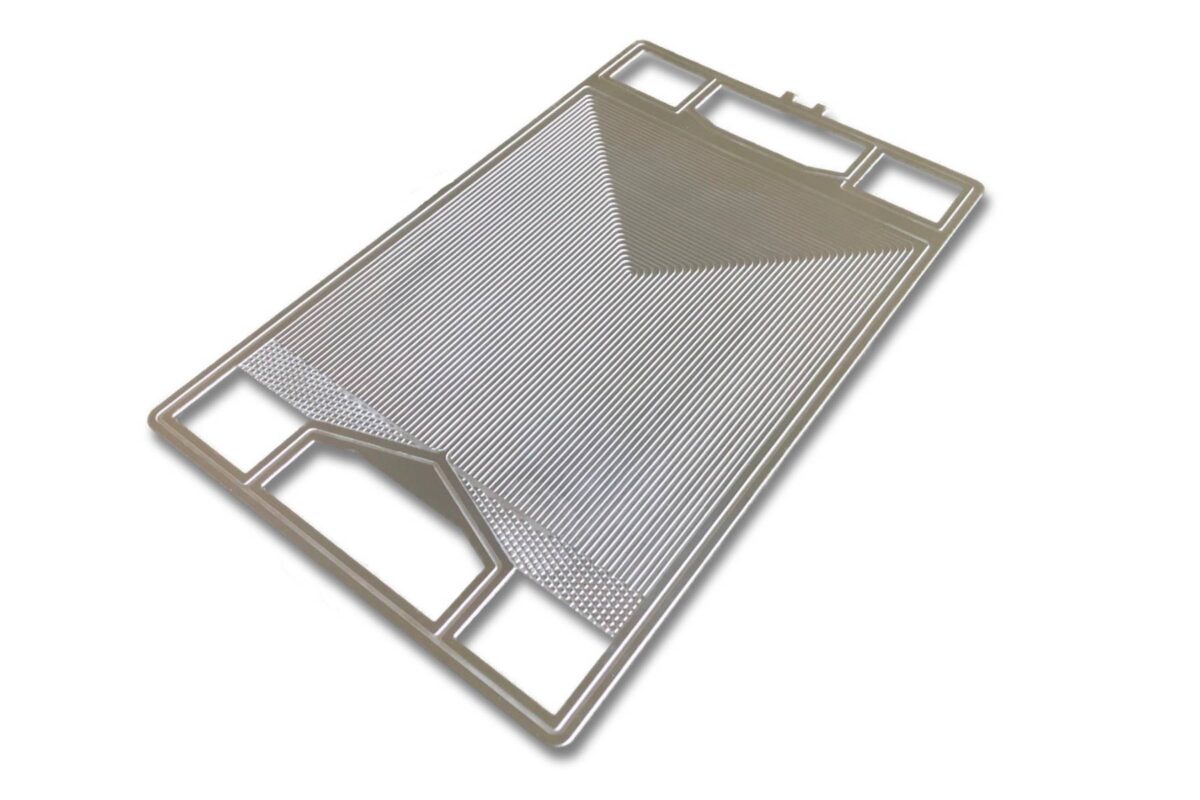
Example: Fuel cell Bipolar plate
How do fuel cells generate electricity?
All types of fuel cell designs generate electricity as power output by way of electrochemical processes for converting a gaseous substance into residual byproducts, freeing up electrons as a result.
An electrical current is generated as the result of a chemical reaction with the oxygen contained in the air. In general, fuel cells are distinguished by the type of fuel they use. The hydrogen-powered fuel cell is today the most prominently used fuel cell type.
Types of fuel cells
Several different fuel cell technologies exist, and some of them use a variety of fuels. Some fuel cell technologies use natural gas or methanol. But today, hydrogen is the most common fuel used in fuel cells due to its high energy density and clean burning properties. As a result, hydrogen fuel cell technologies are today most prevalent.
Some examples of fuel cell technologies with respective fuel types used:
- PEM “Proton Exchange Membrane” fuel cells : Use hydrogen
- Solid oxide fuel cell technology: Can use hydrogen, natural gas, or other fuels
- Phosphoric acid fuel cells: Uses hydrogen
- Alkaline fuel cells: Uses hydrogen or other fuels such as methanol or ethanol
- Molten carbonate fuel cells: Can use hydrogen, natural gas, or other fuels
- Direct methanol fuel cells: Uses methanol as fuel
- Regenerative fuel cells: Can use hydrogen, natural gas, or other fuels
Use cases and applications of fuel cells
The use of fuel cells extends from various hydrogen mobility solutions to a multitude of fuel cell power generation applications.
Standardization of components and modular design makes fuel cell technology universally applicable in all manner of power generation scenarios found in our modern industrialized economies.
Pros and cons: fuel cells, batteries, combustion engines
Fuel cell efficiency
Fuel cells can be more efficient than combustion engines in certain circumstances. They have a higher energy conversion efficiency than internal combustion engines, meaning that they can convert a larger portion of the energy stored in the fuel into useful work.
Are fuel cells more efficient than combustion engines or batteries?
It is important to note that the overall efficiency of a fuel cell system depends on a number of factors, including the type of fuel cell, the operating conditions, and the use of the electricity produced. In some cases, fuel cells may not be as efficient as other technologies, such as batteries, or advanced internal combustion engines.
Are fuel cells rechargeable?
By definition, a fuel cell in and by itself is not “rechargeable”. But some designs combine batteries and fuel cells so as to maximize overall efficiency by making best use of the optimum characteristics of both technologies. The battery acts as a buffer and reservoir for electrical energy generated under low-load or no-load conditions. The resulting fuel cell system might then be deemed to be rechargeable.
In some designs, excess electrical energy generated by the fuel cell is used for electrolysis of the water vapor, which is the residual byproduct of the electrochemical process of power generation. The hydrogen thus generated is stored for future use as fuel in the same fuel cell.
Comparison- advantages and disadvantages
| Advantages of Fuel cells | Advantages of Batteries | Advantages of Combustion Engines |
| High energy density | Lightweight | High power density |
| High specific energy | High specific energy | High efficiency |
| Long lifespan | Long lifespan | Low cost |
| Quick refueling | Quick charging | Widely available |
| Zero emissions | Zero emissions |
| Disadvantages of Fuel cells | Disadvantages of Batteries | Disadvantages of Combustion Engines |
| High cost | Low energy density | High emissions |
| Complex infrastructure | Limited charge/discharge cycles | Limited efficiency at low loads |
| Limited hydrogen availability | Low specific energy |
Fuel cells – pros and cons regarding safety and the environment
Are fuel cells bad for the environment? The answer to this question is of paramount importance given that fuel cell technology is deemed to be the optimum solution for building a sustainable economy utilizing renewable energy. The precise answer depends on which type of fuel cell is used.
Damage to the immediate environment by way of emissions can be ruled out in the case of hydrogen fuel cells, because this technology only emits water vapor. But the way hydrogen fuel is produced also plays a role. When comparing so-called “grey” hydrogen obtained from fossil fuels, “blue” hydrogen produced in combination with carbon-capture technologies, and “green” hydrogen generated by leveraging renewable energy technologies, then a categorical “No” to this question can be stated only for the last option: Green hydrogen is the ultimate environmentally-friendly fuel.
Are fuel cells safe?
It will be obvious and self-explanatory that the handling of gaseous flammable fuel such as hydrogen that is used in fuel cells of various types and designs requires sophisticated and dedicated safety systems. Managing the risks of fuel cells inherent in the technology is one of the challenges to be overcome in the course of its widespread implementation.
The dangers of fuel cells are well understood and have already been managed on an itemized basis, and also in various combinations, in other technologies and industries. Industry players are confident that the engineering challenges posed by fuels used and materials required can be overcome to isolate and control safety hazards.
Last update: 15.02.2023