- What is the main function of a hydrogen electrolyser?
- Which chemical reaction happens in a hydrogen electrolyser?
- What are the main types of electrolysers?
- How efficient are hydrogen electrolysers?
- What are the main use cases of hydrogen electrolyser?
- What are the interfaces connected with the electrolyser system?
- List of certain suppliers of hydrogen electrolysers
Bowse all electrolysers from leading suppliers on our marketplace!
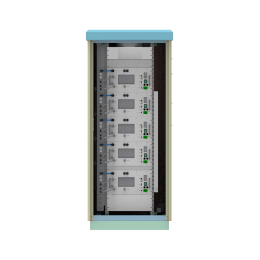
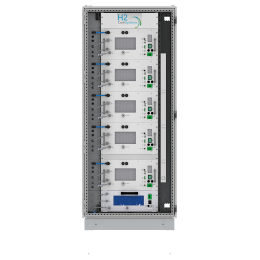
In simple terms, a hydrogen electrolyser, (which can alternatively be written as electrolyzer) is a hydrogen producing device. A hydrogen electrolyzer is a device which splits water into hydrogen and oxygen using electrical energy in a process known as electrolysis. The electrolyser design consists mainly of anode, cathode, membrane/separator, frames, sealing and bipolar plates. A number of cells are connected in series in an electrolyser stack, which can produce more hydrogen. The efficiency of electrolysers varies depending on the type of electrolyser and its operating conditions.
There are different electrolyzer technologies available on the market. To connect with electrolyzer manufacturers and electrolyzer companies in the future, please visit hyfindr.com – the marketplace for the hydrogen economy.
Use of hydrogen is an opportunity to change the market in the energy industry. As our planet is undergoing climatic changes, hydrogen as a fuel enables low emissions and less damaging impact on the planet. Generation of hydrogen using electrolyser technology enables a fully sustainable energy cycle.
What is the main function of a hydrogen electrolyser?
Water electrolysers are used to produce hydrogen by splitting water with the help of electricity. Such a hydrogen electrolyser uses electricity, which passes though electrodes dipped in water. Depending upon the type of electrolyser the water can be mixed with other solutes. The hydrogen and the oxygen can be stored in tanks and can be used later.
The hydrogen generator, called electrolyser, is a key component for making the hydrogen economy work.
Which chemical reaction happens in a hydrogen electrolyser?
The hydrogen electrolyzer converts electrical energy (electricity) into chemical energy (water is split into hydrogen and oxygen). Based on this principle, the electrolyzer equation is as follows. The equation can change depending upon the type of electrolyser used.
At anode: 2 H2O → 4 H+ + O2 + 4e–
At cathode: 2 H+ + 2 e– → H2
In total: 2H2O → 2H2 + O2
What are the main types of electrolysers?
Electrolyzers are available in a variety of designs, depending on the electrolyzer technology used.
1. Polymer electrolyte membrane (PEM) electrolyser
In the PEM electrolyzer design, the electrodes are separated by a solid electrolyte i.e. PFSA (Perfluoroacidsulfonic) membrane, which allows only the passage of protons through it. This PFSA membrane is chemically and mechanically robust. It can withstand a pressure of 70 bar and temperatures of 50 – 80°C. A titanium-based material is used as a substrate covered by a metallic catalyst layer and a protective coating offering long-term stability to cell components, optimal electron conductivity and cell efficiency. A hydrogen PEM electrolyzer uses a thin 0,02 mm PFSA membrane and electrodes for higher efficiencies. Given that rare earth minerals and precious metals are needed for its manufacture, this type of electrolyser design is relatively more expensive than an alkaline electrolyser.
PEM electrolyzers are simple, effective and compact, but sensitive to water impurities. The electrode surface area has reached up to 2000 sq. cm. The PEM electrolyser efficiency is about 60-70% depending on the working conditions.
2. Alkaline electrolyser
This type of electrolyser has a simple stack and a design which makes it comparatively easy to manufacture. It works using a concentrated (25-30%) potassium hydroxide (KOH) solution as the electrolyte, robust diaphragms based on ZrO2 and nickel-coated stainless steel for electrodes.
Typical operating temperatures and pressures are 70 – 90° C and 1 – 30 bar, respectively. Some variants of alkaline electrolysers have recorded a lifetime above 30 years. The efficiency of an alkaline electrolyser is approximately 60 – 70%, depending on the prevailing operating conditions.
3. Anion exchange membrane (AEM) electrolyzer
The AEM hydrogen electrolyser offers a combination of the benefits of a less harsh alkaline electrolyzer with the better efficiency of PEM electrolyzers. Compared to the other types described in this article it is one of the more recently developed technologies, and thus has a growing application scope. In the design of Anion Exchange Membrane Electrolysers, high surface area nickel or NiFeCo alloys are used as a catalyst. The anode and cathode are usually made of nickel foam or carbon cloth. The AEM hydrogen electrolyser does not require precious metals such as titanium and therefore allows for lower cost solutions. It operates under different pressures of up to 35 bar and temperatures between 40 – 60°C. Whilst improvements are still expected in some characteristics such as the efficiency of the electrolyser, very promising commercial solutions are available.
4. Solid oxide electrolyser cell (SOEC)
In a solid oxide electrolyzer (SOEC), low cost Nickel electrodes are used in combination with the electrolyte of which a common example is Yttria-stabilized Zirconia (YSZ). The catalyst used on the oxygen side is of the Perovskite-type (LSCF, LSM, etc.) while on the hydrogen side Ni/YSZ is used. The typical working temperature of SOEC is in the range of 700 – 850°C with the pressure at 1 bar. The efficiency of SOEC can get up to 90% while using more heat energy instead of electrical energy to split water. Its use is most attractive to applications where excess heat is available.
SOECs can be found in the areas of fuel production, carbon dioxide recycling, chemical synthesis and syngas. Applications are typically at kW scale with some projects reaching 1 MW.
Electrolyser technology has evolved into a variety of distinct and mature systems, each offering its own set of tangible benefits.
How efficient are hydrogen electrolysers?
The electrolyzer efficiency calculation is based on kilowatt hours consumed per kilogram of hydrogen produced, i.e. kWh/kg(H2). Comparing this to the amount of energy contained in 1kg of hydrogen allows for the efficiency of an electrolyser to be calculated.
For example, if the hydrogen electrolyser consumes 50kWh of electricity to produce 1kg of Hydrogen, then the efficiency is determined by dividing the energy consumed by the higher heating value of hydrogen (39.4 kWh/kg). This yields an efficiency of 79%.
There are many factors affecting the overall efficiency and the durability of the electrolyzer. Some major factors affecting the efficiency of electrolyzers are gas permeation, electrodes, nickel alloys, water impurities, operating conditions, anode dissolution and variable load. While considering the overall efficiency of the electrolyzer, several other elements need to be taken into consideration. These are the coolers, purifiers, water and thermal management, and other power consumers of the entire electrolyzer system.
What are the main use cases of hydrogen electrolyser?
- Backup power system – electrolyser with fuel fell, hydrogen storage, battery and other components can be used as backup power supply to remote areas, power grids, etc.
- Hydrogen and oxygen production – the hydrogen is used as a fuel and oxygen can be used in many fields like medical applications, steel production, flat glass plants, or the semi-conductor industry.
- Power for mobility applications – hydrogen produced on site can be used at refueling stations for fuel cell electrical vehicles.
- Domestic and industrial power – Hydrogen produced can be injected into natural gas pipeline networks to lower the carbon footprint of heating applications.
- Fuel – It can be used to remove sulphur from fossil fuels, and mixed with other fuels to increase their efficiency.
- Generation of green hydrogen which in turn can be used to produce green chemicals, synthetic fuels, jet fuel, methanol, or industrial goods like fertilizers, etc.
What are the interfaces connected with the electrolyser system?
The main components that are directly/indirectly connected to the electrolyser system in different applications are stated as below.
- Fuel cell systems
- Batteries
- PV grid
- Thermal control system
- Water supply system
- System control
- Hydrogen storage
- Oxygen storage
- Pressure control systems
- Pumps
- Filters
- Electric power input
- Power management
- Exhaust system
List of certain suppliers of hydrogen electrolysers
These manufacturers offer hydrogen electrolysers:
- AFC Energy
- Areva H2Gen
- Clean Power Hydrogen Group
- Elogen
- Enapter
- ErreDue
- Green Hydrogen Systems
- H2 Core Systems
- H2-NRG
- H-Tec Systems
- Hydrogen Pro
- iGas Energy
- IPS-Fest
- ITM Power
- John Cockerill
- Hydrox Holdings
- Linde
- McPhy
- Nel
- PlugPower
- Pure Energy Centre
- Siemens Energy
- Sunfire
- Thyssenkrupp Uhde Chlorine Engineers
Last update: 15.1.2023